electronic configuration of tin|Tin, electron configuration : Tagatay Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the . Tingnan ang higit pa Amourette 300 W X - Kvaliteetne pesu tunnustatud brändidelt Triumph, Sloggi, Damella, HOM. Sinu pesu. Sinu lähedal.
PH0 · WebElements Periodic Table » Tin » properties of free
PH1 · Tin, electron configuration
PH2 · Tin electron configuration
PH3 · Tin Electron Configuration (Sn) with Orbital Diagram
PH4 · Tin (Sn)
PH5 · Tin
PH6 · Electron configuration for Tin (element 50). Orbital diagram
PH7 · Electron Configuration for Sn, Sn 2+, and Sn 4+
PH8 · Complete Electron Configuration for Tin (Sn, Sn2+, Sn4+)
PH9 · Chemistry of Tin (Z=50)
r/Osana. r/Osana. A subreddit for Yandere Simulator! (sans the censorship of its creator!!) Members Online. Y'all down bad as hell but here it is anyway 2. upvotes .
electronic configuration of tin*******Atoms can jump from one orbital to another orbital in an excited state. This is called quantum jump. The ground-state electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. In the tin ground-state electron configuration, the last electrons of the 5p orbital are located in the 5px . Tingnan ang higit paThe total number of electrons in tin is fifty. These electrons are arranged according to specific rules in different orbitals. The arrangement . Tingnan ang higit pa
Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the . Tingnan ang higit pa
The electron configuration shows that the last shell of tin has four electrons. Therefore, the valence electrons of tinare four. . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable . Tingnan ang higit paelectronic configuration of tin Electron Configuration of Tin. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (II) . To write the configuration for the Tin (Sn) and the Tin ions, first we need to write the electron configuration for just Tin (Sn). We first need to find the number of .electronic configuration of tin Tin, electron configuration Tin Electron Configuration. It shows a chemical similarity to both of its neighbors that are germanium and lead in group 14 and has two main oxidation states, that is +2 and the little more stable +4.Sn (Tin) is an element with position number 50 in the periodic table. Located in the V period. Melting point: 232 ℃. Density: 7.29 g/cm 3 .
Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. . Aufbau principle. First, find electrons of tin atom. Periodic table | Image: Learnool. The atomic number of tin represents the total number of electrons of tin. Since the atomic number of tin is 50, the .Electron configuration for tin. The history of Tin. Periodic table history. Identifiers. List of unique identifiers for Tin in various chemical registry databases. Tin is a chemical .Tin. Full electron configuration of tin: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 2. indium ← tin → antimonyTin atoms have 50 electrons and the shell structure is 2.8.18.18.4. The ground state electron configuration of ground state gaseous neutral tin is [ Kr ]. 4d10. 5s2. 5p2 and the term symbol is 3P0. Schematic electronic .Element Tin (Sn), Group 14, Atomic Number 50, p-block, Mass 118.710. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs.The ground state electron configuration of ground state gaseous neutral tin is [Kr].4d 10.5s 2.5p 2 and the term symbol is 3 P 0. Schematic electronic configuration of tin. The Kossel shell structure of tin. .Note that these electron configurations are given for neutral atoms in the gas phase, which are not the same as the electron configurations for the same atoms in chemical environments. In many cases, multiple configurations are within a small range of energies and the irregularities shown below do not necessarily have a clear relation to .
Protons and Neutrons in Tin. Tin is a chemical element with atomic number 50 which means there are 50 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.In this work, efficient electronic configuration modulation of SnO 2 nanoparticles was realized by non-metallic phosphorus (P) doping, giving rise to the considerable transformation into a four-electron transfer-governed water oxidation reaction with the electrocatalytic activity even comparable to the benchmark RuO 2 nanoparticles. These . The electronic configuration of the Tin atom 1s 2, 2s 2, 2p 6, 3s 2, 3p 6, 4s 2, 3d 10, 4p 6, 5s 2, 4d 10, 5p 2 consists of 50 electrons and is known as post-transition metal present in the 14th group of P- blocks in the periodic table. Tin is also used in alloy formation as it has two allotropes and a diamond cubic structure.
The electron configuration of lead is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d10 6s2 6p2, if the electron arrangement is through orbitals. . In the tin ground-state electron configuration, the last electrons of the 6p orbital are located in the 6p x and 6p y orbitals. Now the atomic number of Tin (Sn) is 50. Hence the electron arrangement in Tin is 2, 8, 18, 18, 4. And the electron configuration of Tin is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 2. This electron arrangement and electron configuration indicates that the outermost orbit (i.e orbit number 5) of Tin atom has 4 electrons.
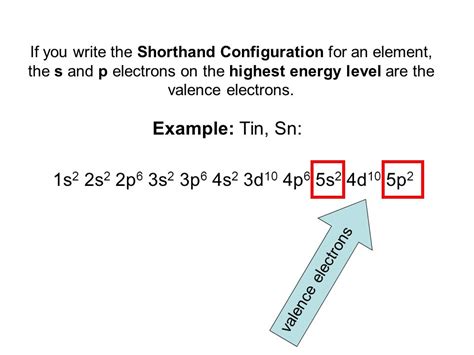
The noble gas configuration is a shorthand electron configuration for atoms. In chemistry, the noble gas configuration is a shorthand method of writing an atom’s electron configuration.The reason for using the noble gas configuration is because the full electron configuration becomes very long for atoms with high atomic .Tin, electron configuration The noble gas configuration is a shorthand electron configuration for atoms. In chemistry, the noble gas configuration is a shorthand method of writing an atom’s electron configuration.The reason for using the noble gas configuration is because the full electron configuration becomes very long for atoms with high atomic .

Electronic configuration of the Tin atom in ascending order of the levels: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 2 Reduced electronic configuration Sn: [Kr] 4d 10 5s 2 5p 2. Below is the electronic diagram of the Tin atom Distribution of electrons over energy levels in the Sn atomIn this work, efficient electronic configuration modulation of SnO 2 nanoparticles was realized by non-metallic phosphorus (P) doping, giving rise to the considerable transformation into a four-electron transfer-governed water oxidation reaction with the electrocatalytic activity even comparable to the benchmark RuO 2 nanoparticles. These .
Electronic configuration of Tin:- Atomic number of Tin is 50, that is Tin has a total of 50 electrons. Since 1s orbital can hold a maximum of 2 electrons, so first 2 electrons will fill the 1s orbital, and the next 2 electrons will fill the 2s orbital.
The electron configuration of tin is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2. Learn how to find: Tin electron configuration. Now in the next step, start drawing the orbital diagram for tin. Draw orbital diagram. Before drawing the orbital diagram, you should know the three general rules.The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z. Electron configuration of Tin is [Kr] 4d10 5s2 5p2. Possible oxidation states are +2,4.
Similarly, the observed electron configuration of copper is [Ar]4s 1 3d 10 instead of [Ar]s 2 3d 9. The actual electron configuration may be rationalized in terms of an added stability associated with a half-filled (ns 1, np 3, nd 5, nf 7) or filled (ns 2, np 6, nd 10, nf 14) subshell. Given the small differences between higher energy levels .
This tin ion(Sn 2+) has fifty protons, sixty-nine neutrons, and forty-eight electrons. Also, tin has one more ion. That is Sn 4+. Sn – 4e – → Sn 4+ Here, the electron configuration of tin ion(Sn 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10. This tin ion(Sn 4+) has fifty protons, sixty-nine neutrons, and forty-six electrons. Tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic structure.The chemical symbol for Tin is Sn. Electron Configuration and Oxidation States of Tin. Electron configuration of Tin is [Kr] 4d10 5s2 5p2. Possible oxidation states are +2,4. Electron Configuration. The .The electron configuration for tin is: {eq}{[Kr]}4p^{10}5s^25p^2 {/eq} The Kr in brackets is the element krypton. It is a noble gas, which means it. See full answer below. Become a member and unlock all Study Answers. Start today. Try it .
Lost or forgotten iPhone passcode? Help is at hand; here's how to bypass a forgotten passcode on iPhone or iPad. Products ; Download; Store; Support; Resources; Data Recovery . The purpose of this tutorial is to help you bypass forgotten passcode on your own phone - 4 digit code or 6 digit code. Before we begin, it's worth checking that .
electronic configuration of tin|Tin, electron configuration